| Source |
Tuesday, May 10, 2016
Airbag lab
Dylan and I did this lab together, as we had the same bag. We checked the math many times over but we still failed. I still feel awful about getting a zero on a lab so I don't really want to talk about it much
Sunday, May 8, 2016
Preparing for the end of the course
Here's some final links on Gas Laws. The end is in sight, my friends.
 |
| Source |
Quiz
For the weekly quiz I prepared by doing the lecture quizzes and watching videos. I need to prepare for more conceptual stuff though
Gas Laws Lesson 1, 2, 3
These laws deal with
Pressure= P
Volume= V
Temperature= T
Moles= n
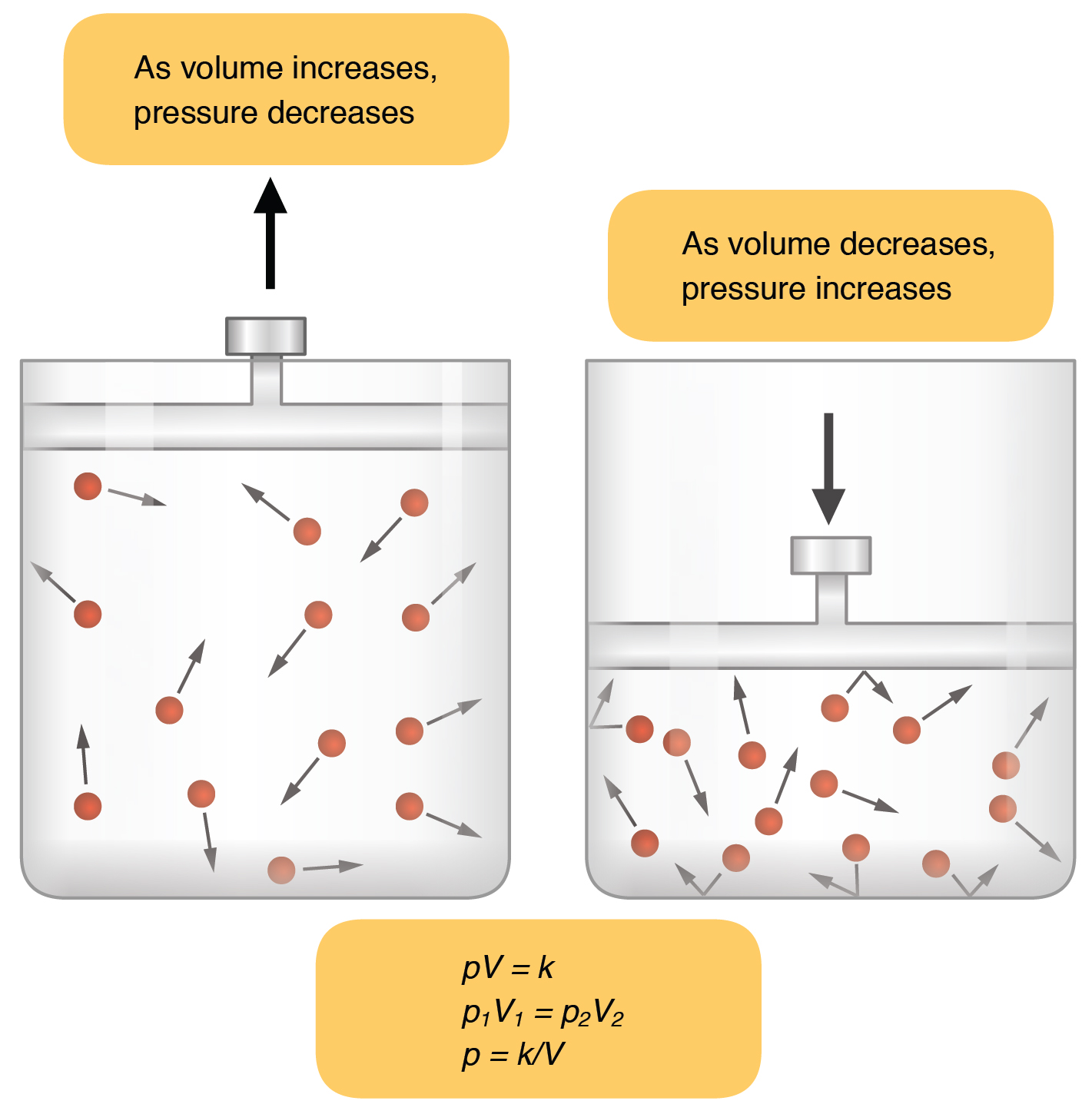
Boyles Law: Temperature is held constant
Lesson
Charles Law: Pressure is held constant
Lesson
Avogardo Law: (MOLES!) Temperature and pressure are held constant (at STP)
Lesson
Pressure= P
Volume= V
Temperature= T
Moles= n
Boyles Law: Temperature is held constant
Lesson
 |
| Source |
Charles Law: Pressure is held constant
Lesson
 |
| Source |
Avogardo Law: (MOLES!) Temperature and pressure are held constant (at STP)
Lesson
 |
| Source |
Thursday, April 28, 2016
Wednesday, April 27, 2016
Energy Test
I don't think the test was too bad! Despite having only 28 minutes for 20 questions, everyone finished. The lecture test were super helpful. I am kicking myself though because I over thought a couple of questions that I knew, which was annoying. But I am still happy with how it went!
Friday, April 15, 2016
Biodiesel Boat Races
This week we made our own biodiesel using Old Chick-fil-a oil. Which was absolutely disgusting and made me sick. But Dylan and I's biodiesel lasted in the cup and we were able to use it to run our surfboard boat (The Silver Surfer). The Silver Surfer met our expectations of being the slowest boat to cross the finish line! We are proud.
Some links to help with the project:
How to Make Biodiesel
Why using Biodiesel is better
Biodiesel Engine
Putt Putt Engine
Video:
Guideline
Submitting
Some links to help with the project:
How to Make Biodiesel
Why using Biodiesel is better
Biodiesel Engine
Putt Putt Engine
Video:
Guideline
Submitting
| Source |
Tuesday, April 5, 2016
Biodiesel video
| Source |
Unfortunately I do not have the link to Dylan's video since I'm in New York, but I saw it and it looks great! I'm glad he was able to finish it on his own, I feel awful about it.
| Source |
What is Biodiesel?
I've learned a lot about biodiesel in these few days researching it for Dylan and I's video. I think Biodiesel.org explained it best:
"Biodiesel is a renewable, clean-burning diesel replacement that is reducing U.S. dependence on foreign petroleum, creating jobs and improving the environment. Made from a diverse mix of feedstocks including recycled cooking oil, soybean oil, and animal fats, it is the first and only EPA-designated Advanced Biofuel in commercial-scale production across the country and the first to reach 1 billion gallons of annual production. Meeting strict technical fuel quality and engine performance specifications, it can be used in existing diesel engines without modification and is covered by all major engine manufacturers’ warranties, most often in blends of up to 5 percent or 20 percent biodiesel. It is produced at plants in nearly every state in the country."
Now we start making a video
"Biodiesel is a renewable, clean-burning diesel replacement that is reducing U.S. dependence on foreign petroleum, creating jobs and improving the environment. Made from a diverse mix of feedstocks including recycled cooking oil, soybean oil, and animal fats, it is the first and only EPA-designated Advanced Biofuel in commercial-scale production across the country and the first to reach 1 billion gallons of annual production. Meeting strict technical fuel quality and engine performance specifications, it can be used in existing diesel engines without modification and is covered by all major engine manufacturers’ warranties, most often in blends of up to 5 percent or 20 percent biodiesel. It is produced at plants in nearly every state in the country."
Now we start making a video
Thursday, March 17, 2016
More Lectures
Chemical bonding revolves around one concept: a covalent bond is formed by sharing electrons. We do not focus on ionic bonding too much in this unit. Atoms want electrons to fill their valence shells to make them stable. More often than not, it's 8 electrons to fill a valence shell except for Be, B, H, & He. We learned lewis structures, formal charge, electron geometry.
| Source |
 |
| Source |
Atomic Model lab
Basically this lab was about practicing have need share. I've been having a lot of struggle focusing so this stuff is still lost on me. I'm trying though
 |
| Source |
Tuesday, March 15, 2016
Helpful links on bonding lessons
Here's some links that helped me to understand The Chemical Bonding Lessons
Molecular geometry
Lewis Structure notes
Bonding Notes
Molecular shape theory video
Formal Charge and Dot Structure theory
Good luck on the test/quizzes everyone!
Molecular geometry
Lewis Structure notes
Bonding Notes
Molecular shape theory video
Formal Charge and Dot Structure theory
Good luck on the test/quizzes everyone!
Sunday, March 6, 2016
Unit Test Thoughts
Well, I don't have the grade back yet... but I think I did pretty okay. I actually got to read all 50 questions, which is rare for any unit test. So I'll count that as a victory.
 |
| Source |
Thursday, March 3, 2016
Periodic Trends
The last lesson for this unit was periodic trends. The periodic table has trends that can provide information about the chemical and electronegative characteristics of S & P block atoms. i believe this concept was very easy to understand as long as you memorize the details.
 |
| Source |
Friday, February 26, 2016
Electronic Structure Quiz
Overall I feel pretty confident about this unit. I don't have a ton of time to study but I'm pretty sure I know the material well. Let's hope for the best! Here's some study stuff:
Electronic Structure Video 1
Electronic Structure Video 2
Periodic Trends Video
Calculating Energy
Electromagnetic Spectrum
Periodic Trends
Electronic Structure Video 1
Electronic Structure Video 2
Periodic Trends Video
Calculating Energy
Electromagnetic Spectrum
Periodic Trends
Wavelength Lab
In this lab we held a spectroscopic analysis with a spectrophotometer, in order to see the wavelengths of chromate and cobalt. We adjusted various knobs on the spec 20 and found the amount of light in blue and pink solutions, which were our metals. This lab was tedious and very boring
Thursday, February 18, 2016
Flame Test Lab
Today we got to play with fire. Basically we took solutions and held them in a flame to determine what color they burned. From the color we can tell wavelength, and then calculate energy. Here so pics of the awesome colors!
Wednesday, February 17, 2016
Electronic Structure
The next unit, Electronic Structure, This unit is more based on concepts than math, unlike our previous units. There is a lot less little variations. We learned about the wave nature of light. We also talked about wavelength, the wave spectrum, frequency, and calculating energy. Hopefully this continues to be easy.
 |
| Source |
Wednesday, February 10, 2016
Acid Base Test
Overall, I feel pretty confident about this test. I feel like this is the first unit in a long time that I understand. I did study quite a lot so I'm really hoping for a good grade. Here's some helpful links!
Acid Base video 1
Acid Base Video 2
Acid Base Equation Video
Online Titration
Acid Base Khan Academy
Acid Base Vision Learning
Acid Base video 1
Acid Base Video 2
Acid Base Equation Video
Online Titration
Acid Base Khan Academy
Acid Base Vision Learning
Tuesday, February 9, 2016
Unknown Acid Lab
More titration, woooo. It's very tedious and time consuming. This lab has basically the same set up as the Acetic acid lab except instead of vinegar, we used an acid with an unknown concentration. The acid was a solid, so first we had to use the hot plate with the stirrer to dissolve it in distilled water. We were able to get a pretty good pink color on most of our titrations, however our average was very low so we had to do many trials.
Monday, February 8, 2016
Acetic Acid Lab 2
This lab we set out to find the percent of acetic acid in common vinegar. We standardized a solution of NaOH with the acid potassium hydrogen phthalate (KHP) in a titration to determine the molarity of NaOH. Next we used the standard NaOH solution to determine the percent of acetic acid in vinegar. Lastly we diluted 10mL vinegar with 90 mL distilled water to make a 100mL solution, add our KHP and titrate as usual. We used this dilution to compare. The lab was pretty fun.
Sunday, February 7, 2016
Acetic Acid lab
We began the Acetic Acid Lab today. This lab means we get to do titrations first hand. Today we just practiced the lab because it's quite tricky. A titration is where a solution of known concentration is used to determine the concentration of an unknown solution. The titrant is added from a buret to a known quantity of the unknown solution until the reaction is complete. In our lab, the titrant was our base, NaOh and the unknown solution is the substance in the flask, KHP and Acetic Acid, The indicator is phenolphthalein, which turns the solution pink when the endpoint is reached. Also phenolphthalein is my new favorite word.
Here's the video Frank showed us in preparation for the lab:
Video
Here's the video Frank showed us in preparation for the lab:
Video
Monday, January 25, 2016
Quiz- Acid Base Thoughts
Wow this feels like the first easy quiz we've ever had. For once I feel like I'm doing chemistry right. A lot of it was doing ice-box and ph calculations which are easy if you memorize the steps.Overall I'm just hoping for a good grade.
Vitamin C Lab
Basically today we learned that Vitamin C is a essential nutrient: we need it to survive. Vitamin C is most commonly found in citrus fruits, but also in various vegetables. Lack of vitamin C can cause disease such as scurvy. Yarr.
In this lab we worked on comparing the vitamin C concentration of different juices to a 1mg/mL standard solution. To do this we added starch to 20 drops of each juice, then added a couple drops of iodine. This reaction will make a clear liquid. However, if you continue to add iodine after all the vitamin c has reacted, the solution will turn blue. More trials to do tomorrow.
In this lab we worked on comparing the vitamin C concentration of different juices to a 1mg/mL standard solution. To do this we added starch to 20 drops of each juice, then added a couple drops of iodine. This reaction will make a clear liquid. However, if you continue to add iodine after all the vitamin c has reacted, the solution will turn blue. More trials to do tomorrow.
Saturday, January 23, 2016
End of Unit + Test Thoughts
Uhhh.. yikes. I did very bad on this test. Like the worst I've done on any test I've ever taken. Whoops. I try not to blame myself because I wasn't there for most of the lessons and it's super hard to teach yourself chemistry.. but yikes this is rough. Bye chem grade.
Thursday, January 21, 2016
Last Lesson for Aqueous Solutions
And for the last lesson of course we tie it all back to Stoic. Basically now we know how to find grams, volume, moles, or molarity of a substance, using molarity (mol per 1L) as a conversion factor. Using this we can find volume to moles and vice versa. Then from there we can go on to convert to grams.
 |
| Conversion Chart Source |
Wednesday, January 20, 2016
Solving a Murder Lab Day 2
Tuesday, January 19, 2016
Solving a Murder Lab Day one
Sunday, January 10, 2016
Chemistry in.... Ice Cream?
Today we read an article on ice cream. Basically it was about the science behind ice cream. Ice cream includes many different factors such as air, fats, and coldness. All of these factors help make ice cream as good as it is. No idea what this has to do with aqueous solutions but I do like ice cream.
 |
| Source |
Subscribe to:
Comments (Atom)












